
| Technetium | |
| Electrons per shell | 2,8,18,13,2 |
| Electron Configuration | [Kr] 4d5 5s2  |
| Ground state | 6S5/2 |
| Atomic Volume | 8.5 cm3 mol-1 |
| Electronegativity | 1.9 |
| Magnetic ordering | No data |
| Mass magnetic susceptibility | 3.42 x 10-8 |
| Molar magnetic susceptibility | 3.352 x 10-9 |
 Thermal Properties Thermal Properties |
|
| Enthalpy of Atomization | 649 kJ mol-1 @25°C |
| Enthalpy of Fusion | 23.81 kJ mol-1 |
| Enthalpy of Vaporisation | 585.22 kJ mol-1 |
| Heat Capacity | 24.27 J mol-1 K-1 |
| Thermal Conductivity | No data. |
| Thermal Expansion | No data. |
 Electrical Properties Electrical Properties |
|
| Electrical resistivity | No data. |
| Electrical conductivity | 0.067 106/cm Ω |
 Chemical Properties Chemical Properties |
|
| Electrochemical Equivalent | 0.51651 g Ah-1 |
| Valence Electron Potential (-eV) | 180 |
| Ionization Potential | |
| 7.28 | |
| 15.26 | |
| 29.54 | |
 Energy Levels Energy Levels |
|
| 18.3671 KeV | |
| 18.2508 KeV | |
| 20.619 KeV | |
| 21.0015 KeV | |
| 20.5947 KeV | |
| 2.424 KeV | |
| 2.5368 KeV | |
| 2.6149 KeV | |
| 2.5963 KeV | |
| 2.249 KeV | |
| 2.1296 KeV | |
 Atomic Radii Atomic Radii |
|
| 135 pm | |
| 183 pm | |
| 156 pm | |
| No data. | |
| 110 pm | |
| 136 pm | |
 Oxidation States Oxidation States |
|
| Tc+4, Tc+5, Tc+7 | |
| Tc-1, Tc0, Tc+6 | |
 Ionisation Energies (kJ mol-1) Ionisation Energies (kJ mol-1)  |
|
| 702 | |
| 1472 | |
| 2850 | |
| 4100 | |
| 5700 | |
| 7300 | |
| 9100 | |
| 15600 | |
| 17800 | |
| 19900 | |
 Vapour Pressure Vapour Pressure |
||||||
| P (Pa) | 1 | 10 | 100 | 1K | 10K | 100K |
| T (K) | 2727 | 2998 | 3324 | 3726 | 4234 | 4894 |
 Crystal Structure Crystal Structure |
|
| Hexagonal close packed | |
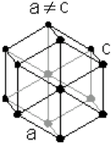 |
a = 273.5 pm |
| b = 273.5 pm | |
| c = 438.8 pm | |
| α = 90° | |
| β = 90° | |
| γ = 120° | |